iTind
Introduction
The iTind procedure is an ideal alternative to prescription medication or invasive surgery. iTind avoids many of the complications associated with these treatment options. Additionally, no permanent implant is left behind resulting from the procedure.
iTind is a minimally invasive and clinically proven treatment that provides rapid and effective relief from BPH symptoms. The treatment involves the implantation of a small device into the prostate for 5-7 days, after which it is completely removed. Once the iTind has been implanted, it expands and applies gentle pressure, remodelling the tissue and creating a wider channel through which urine can flow.
The Benefits of iTind:
- Rapid and effective symptom relief with durable results
- Rapid return to daily life
- No requirement for general anaesthesia
- Routinely catheter free procedure
- No permanent implant resulting from the procedure
- Preserves sexual and ejaculatory function and urinary continence
How is the procedure performed
The iTind procedure will be performed by your surgeon under a short general anaesthetic in the operating theatre as a day case.
Insertion of the iTind: A small thin scope (cystoscope) with a tiny camera will be placed into your urethra tube to determine where to place the iTind. Your surgeon will then place the iTind device in your prostate. Once in place you should be able to urinate freely and you will be released to go home. Routinely there is no need for a catheter.
The Implantation Period: During the next 5-7 days you may return to most normal activities, depending on how comfortable you feel. You may have soreness in the lower abdomen, and it may be uncomfortable to sit. You may experience the need to urinate more frequently and with greater urgency. You may also have some blood in your urine. These are all normal reactions.
The Removal of iTind: After one week, your urologist will completely remove the iTind device using a flexible silicone catheter. You may return to normal activities 1-2 days after the removal.
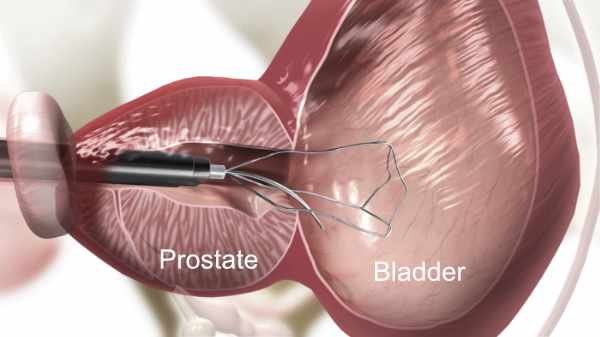
1. iTind Insertion
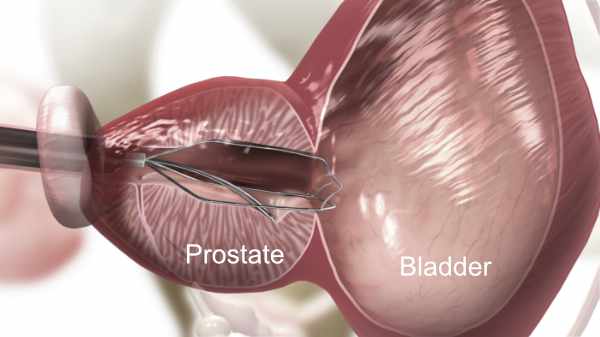
2. iTind Positioning
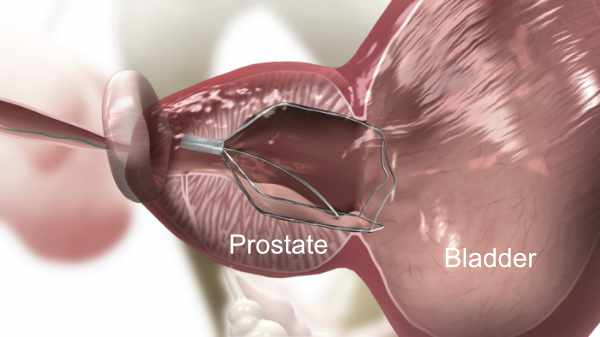
3. iTind Expanded
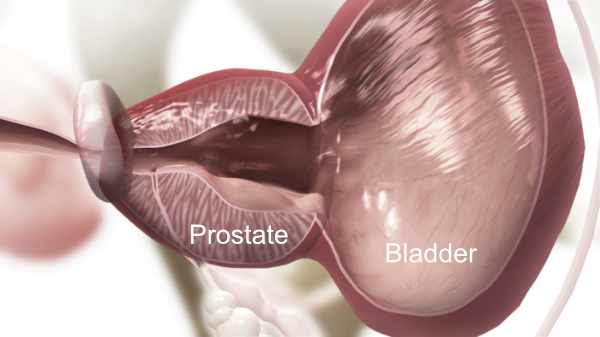
4. iTind Removed
Post-operation advice
Upon returning home you may return to most normal activities depending on how comfortable you feel. Most patients start to feel symptom relief as soon as the device has been removed. Symptoms typically continue to improve over the next 6 to 12 weeks. You will be reviewed by your surgeon over the weeks and months following the procedure who may ask you to perform a urinary flow test and symptom assessment at intervals.
Durability has been demonstrated out to three years in terms of symptom improvement, urinary flow and quality of life in a significant number of patients. The iTind treatment does not preclude retreatment or other BPH treatments, should they be needed or desired in the future.
